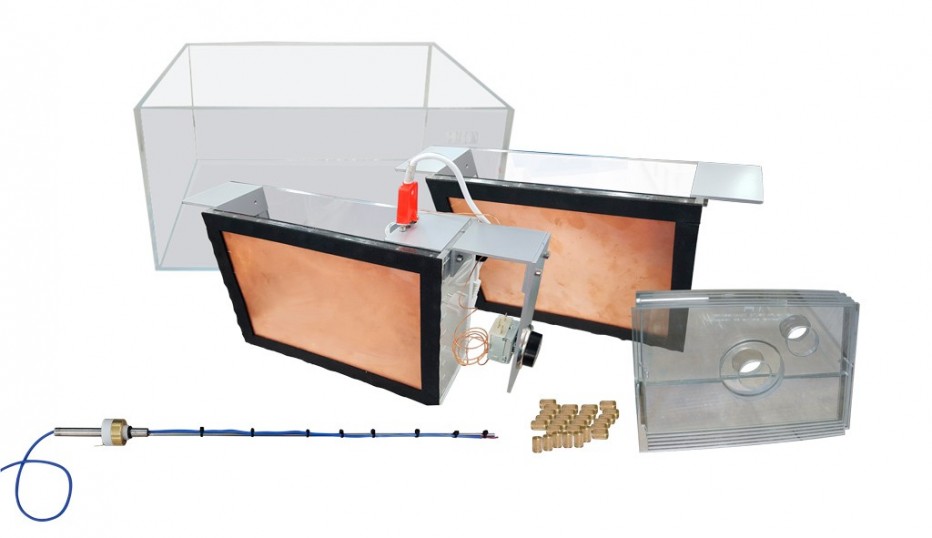
The Set for the Study of Thermodynamics, “FTT”, allows the study of thermodynamics by means of the temperature difference between two sources.
Thermodynamics represents the study of temperature, heat and energy exchange. It has practical applications in all branches of science and technology, as well as in many aspects of daily life, from climate to cooking.
Temperature is familiar to all of us as a measure of how hot or cold bodies are. More precisely, it is a measure of the average internal molecular kinetic energy of a body, but both the definition and determination of temperature is a subtle and complex subject. For example, it is very difficult to define temperature so that different thermometers agree with each other in measuring the temperature of a substance. However, the properties of gases at low densities allow us to define a temperature scale “T” and to construct gas thermometers whose measurements agree.
The transmission of energy from one body to another in a heat exchange process consists simply in the absorption of energy at the molecular level. This energy causes its atoms to increase their degree of excitation, increasing their oscillations in frequency and amplitude. These oscillations, which occur around their equilibrium positions, can even “break” bonds between them, which then become free, causing the element to change its state of aggregation.
At the macroscopic level, temperature translates into the sensation of heat: when we touch an object that is at a higher temperature than that of our skin, we say that it is hot. The temperature difference between two substances will determine the direction of heat flow between them. It is this difference in heat flow that we can study with the Set “FTT”.
SPECIFICATION
Elements:
- Experiment tank.
- Cu tank with resistance inside (hot spot). The resistor will be directly connected to the mains (230 V).
- Cu tank (cold focus).
- Insulating plates: six vertical (250 x 110) and six horizontal (343 x 73).
- Plate fixing nuts.